Introduction
Joint bleeding events (BEs) have cumulative, irreversible and debilitating consequences for persons with hemophilia A or B with inhibitors (PwHABI) due to synovitis and joint iron deposition. To limit the long-term consequences of bleeding into joints, early bleed resolution is a primary treatment goal. Eptacog beta (Sevenfact®, HEMA Biologics and LFB) is a new bypassing agent indicated for the treatment and control of BEs in adults and adolescents with hemophilia A or B with inhibitors. In a prospective, randomized, cross-over, phase 3 clinical trial (PERSEPT 1, NCT#02020369) in the first 24 hours following bleed onset, eptacog beta demonstrated dose-dependent improvements in successful clinical response with 2 initial dose regimens (IDRs) (75 µg/kg IDR: 75 µg/kg q3h; and 225 µg/kg IDR: 225 µg/kg followed by 75 µg/kg q3h after 9 hours if necessary; Figure 1). 91% of all mild or moderate BEs achieved hemostatic efficacy at 12 hours (225 µg/kg IDR), as did 82% at 12 hours in the 75 µg/kg IDR.
The majority (85%) of mild/moderate BEs treated in PERSEPT 1 were joint BEs.
Aims
A subset analysis of mild or moderate joint BE data from the PERSEPT 1 trial was performed to investigate the effect of 2 IDRs on clinical response at 3, 9, 12 and 24 hours on joint BEs in PwHABI. IRB approval and informed consent were obtained.
Methods
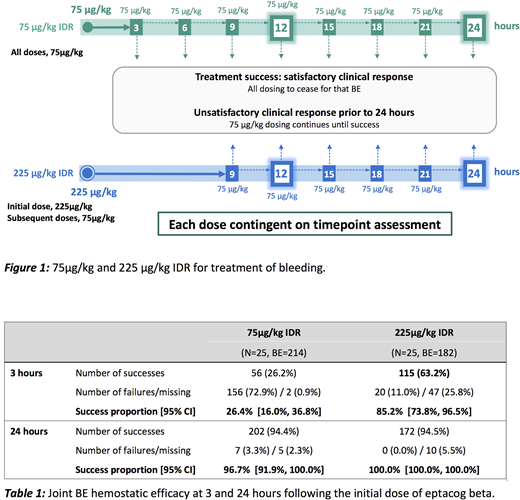
At enrollment, male PwHABI (n=27) were randomized to receive BE treatment on either the 75 µg/kg IDR or the 225 µg/kg IDR (Figure 1) for the first 3 months of treatment; subjects were crossed over to the alternate IDR every 3 months. The 12-hour composite endpoint subset analysis for joint BEs used the same success criteria that were used for the primary efficacy endpoint (for all BEs at 12 hours) in PERSEPT 1. Hemostatic efficacy at all other intermediate timepoints and at 24 hours was assessed using a 4-point evaluation scale (an excellent or good evaluation being considered hemostatic efficacy, and moderate or poor being considered a lack of hemostatic efficacy.) BE treatment continued until bleeding ceased as determined by the subject or physician to discontinue treatment.
Results
A subset of 396 mild/moderate joint BEs were analyzed. The proportion of successfully treated BEs (composite endpoint) at 12 hours was 91.5% [95% CI: 83.4%, 99.6%] on the 225 µg/kg IDR and 80.6% [95% CI: 69.6%, 91.6%] on the 75 µg/kg IDR. At 9 hours, the proportion of BEs with hemostatic efficacy (using the 4-point evaluation scale) from the first dose in the 225 µg/kg IDR was 86.5%; 3- and 24-hour data are shown in Table 1.
Conclusions
The joint BE success proportion following a first dose in the 225µg/kg IDR at 3 hours was 85.2%; joint BEs treated by the 75µg/kg IDR demonstrated a 26.4% 3-hour success proportion. These success proportions demonstrate a dose-dependent onset of action; this effect on efficacy was also observed in the 12- and 24-hour data.
At 9 hours, sustained hemostatic efficacy from a single 225 µg/kg dose was observed (86.5%).
Overall, both initial dose regimens showed successful resolution of joint BEs with early hemostatic success proportions. Further, the onset of action data supports the concept that a larger initial thrombin burst may result in an earlier effective clot that drives earlier bleed resolution. Further observations of this type are needed to understand the role of rFVIIa in hemostatic clot formation.
Hermans:Bayer: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Research Funding, Speakers Bureau; Shire, a Takeda company: Consultancy, Research Funding, Speakers Bureau; Sobi: Consultancy, Research Funding, Speakers Bureau; Biogen: Consultancy, Speakers Bureau; CAF-DCF: Consultancy, Speakers Bureau; CSL Behring: Consultancy, Speakers Bureau; LFB: Consultancy, Speakers Bureau; Novo Nordisk: Consultancy, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Octapharma: Consultancy, Speakers Bureau; Kedrion: Speakers Bureau; EAHAD: Other; WFH: Other. Ducore:Octapharma: Consultancy; Bayer: Consultancy, Honoraria, Speakers Bureau; HEMA Biologics: Consultancy, Honoraria. Escobar:Novo Nordisk: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; National Hemophilia Foundation: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees. Young:BioMarin, Freeline, Genentech/Roche, Grifols, Kedrion, Novo Nordisk, Sanofi Genzyme, Spark, Takeda, and UniQure: Honoraria; Bayer, CSL Behring, Freeline, UniQure: Consultancy; Genentech/Roche, Grifols, and Takeda: Research Funding. Wang:Bayer: Honoraria; Takeda: Honoraria; Genentech: Honoraria; Biomarin: Honoraria; CSL Behring: Honoraria; Bioverativ Inc: Honoraria. Quon:Shire/Takeda: Speakers Bureau; Octapharma: Honoraria; Biomarin: Honoraria, Speakers Bureau; Genentech, Inc./F. Hoffmann-La Roche Ltd: Honoraria, Speakers Bureau; Novo Nordisk: Honoraria, Speakers Bureau; Bayer: Honoraria; Orthopaedic Institute for Children: Current Employment; Bioverativ/Sanofi: Honoraria, Speakers Bureau. Alexander:HEMA Biologics, LLC: Current Employment, Patents & Royalties: No royalties or benefits. Mitchell:HEMA Biologics: Consultancy. Al-Sabbagh:LFB: Current Employment. Bonzo:International Association for Statistical Computing: Other; International Statistics Institute: Other; American Statistical Association: Other; LFB USA, Inc.: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal